Why Do Most Ionic Compounds Dissolve in Water
As potassium chloride KCl dissolves in water the ions are hydrated. Water breaks the ionic bond by hydrogen bonding as water itself has a more ionic bond.

Ionic Compounds And Metals Ppt Download
Ionic compounds are easily soluble in any liquid that is capable of breaking the ionic bond in them.

. While the electrostatic forces of attraction holding an ionic compound together are strong water is its Achilles heel. Ionic compounds dissolve in the presence of water because the positive components of the compound are attracted to the negative charges. Ionic compounds dissolve in water because the hydrogen and oxygen atoms in the H2O molecules have partial charges that attract the ions in the solid compound causing it to dissociate into separated ions.
As potassium chloride KCl dissolves in water the ions are hydrated. Ionic compounds can conduct electricity when they are dissolved in water or in liquid form. The first example that springs to mind is sodium chloride.
Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Differences in electronegativity account for the partial positive. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the.
Most ionic compounds dissolve in water because the process is thermodynamically favourable and kinetically accessible. Entropy causes everything to become more disordered and so ionic compounds will dissolve to increase entropy. Their ions will dissociates and this make tgemto freely move from one place to.
The polar water molecules are attracted by the charges on the K and Cl ions. Water molecules can attract most ions very well luring the. Each ion can participate in multiple ion-dipole interactions when dissolved and these multiple ion-dipole interactions could potentially store more energy than.
These attractions play an important role in the dissolution of ionic compounds in water.

Why Do Ionic Compounds Dissolve In Water Cbse Tuts
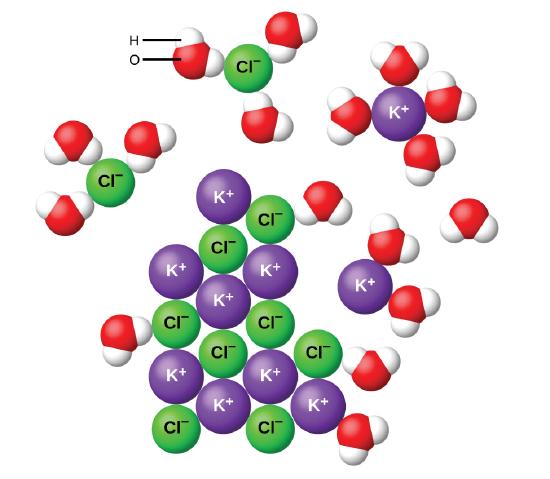
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts


Comments
Post a Comment